.png)
Composition of Feeds, Forages and their Meaning for Small Ruminants
By Basil Bactawar, UF/IFAS Duval County Extension Service
INTRODUCTION
The logical starting point for a discussion on animal feeding is the feed itself. Although some producers raise small ruminants because of their fondness for the animals, in most parts of the world they are raised because of their ability to utilize an otherwise unusable resource to produce food and fiber suitable for human consumption. Small ruminants are viewed merely as forage converters.
In fact, ruminant animals are relatively inefficient in their conversion of feed to human food. The production of beef, chevon and lamb can only be economical when the costs of raw materials are low. For this reason small ruminant production is based on forage, either fresh, as pasture, or conserved, in the form of hay or silage. Except in the case of fast growing lambs and goats, grain and other concentrates should only be viewed as supplements. These feeds are more efficiently utilized either by humans directly or through other animal production systems such as swine and poultry.
FEED COMPOSITION
The Growth of Forages
Forage growth is summarized in Figure 1. All green plants absorb carbon dioxide (C02) from the air through their leaves. Water (H20), nitrates and minerals are assimilated from the soil by the roots. Sunlight, which is trapped by the green pigment chlorophyll, provides the energy which is used by the plant to make carbohydrates, proteins and other organic constituents from these simple nutrients.
Carbohydrates
Carbohydrates are combinations of carbon dioxide and water. The simplest combinations are called sugars and include glucose, galactose, fructose and pentose. These substances are soluble in water and are readily transported through the plant to provide for a variety of requirements. Simple sugars are the building blocks for more complex carbohydrates such as cellulose, hemicellulose and starch.
Cellulose and hemi-cellulose are important constituents of the plant cell wall. Hemicellulose and starch are some of the forms in which plants store energy. These are often referred to as carbohydrate reserves. Starch is the carbohydrate which forms the kernel of feed grains.
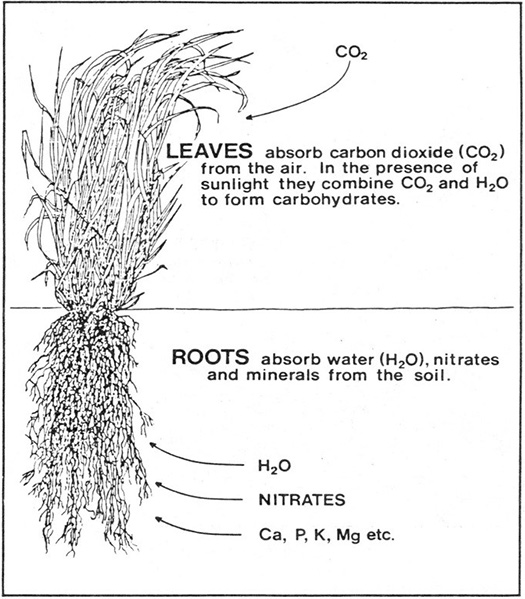
Figure 1. Forage Growth
Fats and Oils
Like carbohydrates, fats and oils are made up of carbon, hydrogen and oxygen. However, the proportion of carbon and hydrogen is much greater with the result that fats and oils furnish 2.25 times as much energy per kilogram as do carbohydrates and proteins.
Plants, except for the oilseeds, contain relatively low levels of fats and oils. Unlike animals which store energy as fat, plants store energy as carbohydrate, as suggested above.
Lignin
The more fibrous parts of plants contain considerable amounts of lignin which is a very complex substance, somewhat similar to the complex carbohydrates. It is important in giving plants some of their structural properties, but is of little value as a feed constituent.
Proteins
In addition to carbon (from CO2), hydrogen (from H2O) and oxygen (from both), plant proteins contain nitrogen and many also contain sulfur and phosphorus assimilated from the soil. These compounds are almost infinite in nature mainly functioning in plants in the form of enzymes.
Proteins are predominantly found in the reproductive parts of plants and in actively growing portions such as leaves. In animals, of course, protein is found mainly in the form of muscle tissue. Of particular importance in sheep production, wool is also composed of protein. Since these constitute the end-products of the sheep enterprise, it is easy to appreciate the importance of protein in the feed.
Legume forages generally contain higher levels of protein than the grasses. This is due to the large supply of nitrogen available to them through fixation from the atmosphere. Nitrogen fixation is facilitated by bacteria contained in nodules on the roots which transfer the nitrogen to the plant (Figure 2). In exchange, the legume plant supplies soluble carbohydrates which provide energy to the bacteria.
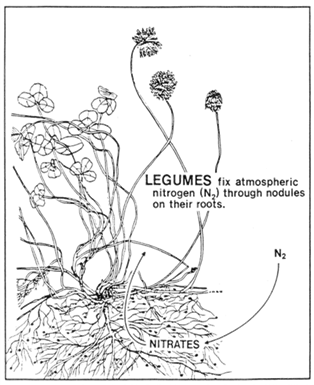
Figure 2. Nitrogen fixation in legumes.
Minerals
Plants assimilate minerals from the soil through their roots. Although present in relatively small amounts, they are as essential to the development of the plant as they are to the animals which consume them. Minerals are well distributed in the plant, largely occurring in association with the organic compounds (carbohydrates, proteins, fats and oils). For example, magnesium is an essential component of chlorophyll. However, it is important to provide a complete, preferably loose mineral to small ruminants. Sheep cannot tolerate high levels of copper; they must be fed minerals supplements formulated for sheep.
Vitamins
Like the other organic compounds, plants synthesize vitamins from raw materials absorbed through their roots and leaves. Some of these, such as the B-vitamins, serve much the same functions in plants as they do in animals. Others serve a specific function in plants which is quite different from their function in animals. For example, carotene is a yellow plant pigments which plays a part in photosynthesis. Animals convert carotene to vitamin A which is essential in maintenance of tissue surfaces. In general, microorganisms in the intestine of sheep and goats produce all the necessary vitamins for the animals except Vitamin A, E and D.
FEED ANALYSIS
Chemical analysis of feed is aimed at estimating its nutritional value for livestock. The analyses have their limitations and a brief description of the methods used will provide an understanding of how the results can be used to best advantage
Dry Matter
The amount of moisture contained in feeds is widely variable. Hay and grain usually contain about 10% moisture. Silage may contain 50-75%. Pasture plants are often 80-85% water. In most feeding situations, animal intake is limited only by the dry matter content of a feed. In other words, a doe who is capable of consuming 2kg (4.4 lbs.) of leafy grass hay (10% moisture: 90% dry matter) will also be capable of consuming 9 kg (19.8 lbs.) of leafy grass pasture (80% moisture: 20% dry matter). In both cases she will consume 1.8kg (4 lbs.) of dry matter. Expressing feed analysis, animal intake and nutrient requirements on a dry matter basis eliminates moisture as a variable in the comparison of different feeds and in the calculation of balanced rations.
Dry matter content (%DM) may also provide information about the storage properties of feed. Extra moisture may result in heating and spoilage in hay and grain. Inadequate moisture in silage may result in poor preservation while high moisture content may lead to excessive nutrient leaching. However, for these purposes dry matter content should be measured before the feed is stored. On the feed analysis report, dry matter is expressed as a percentage. The figure is derived by simply weighing a sample of feed before and after drying at 70-80o C:
| % DM = | dry weight -------------- wet weight |
x 100 |
Fibre (ADF)
ADF stands for acid detergent fiber. This analysis estimates the amount of low digestibility material in a feed by boiling a weighed sample in an acid detergent solution. The residue which remains undissolved consists primarily of lignin, cellulose which is associated with it, and silica. Cellulose digestibility is dependent on the degree to which it is lignified.
| % ADF = | residue -------------- original sample |
x 100 |
Total Digestible Nutrients
TDN is used as an estimate of the energy content of feed, since any of the organic constituents of feed, including protein, will provide energy when digested. Other areas of North America use Digestible Energy (DE) while the United Kingdom, New Zealand and Australia use Metabolizable Energy (ME) in the description of feed energy. These three sets of terminology are simply related for practical purposes as follows:
DE (in megacalories/kg) = % TDN x .044
ME (in megajoules/kg) = % TDN x .15
Protein
Protein contains a significant proportion of nitrogen, setting it apart from the other organic constituents found in feeds. On this basis, the chemical analysis for protein (the Kjeldahl method), measures only the total amount of nitrogen in a sample. The crude protein (CP) level is then calculated, knowing that protein contains average 16% nitrogen:
| % CP = | % nitrogen -------------- 0.16 |
Although this method accurately measures nitrogen, the extrapolation to crude protein is subject to error. Proteins in different feeds vary in their nitrogen content as shown in table A1. In addition, some of the nitrogen present may not be associated with available protein as in:
1. heavily fertilized pastures;
2. forages which have accumulated nitrates;
3. mouldy hay; and
4. heat damaged silage, hay or grain.
TABLE1. The percentage of nitrogen in proteins from various feeds.
| Protein Source | % Nitrogen in Protein |
|---|---|
| Forage Leaves………………….. | 15.0 |
| Barley Grain……………………… | 17.2 |
| Corn Grain………………………… | 16.0 |
| Oilseed Meals…………………… | 18.5 |
| Fishmeal…………………… | 16.0 |
| Milk……………………… | 15.8 |
The level of unavailable nitrogen in heat damaged forages can be estimated using a combination of two analyses which have been described above.
The residue from the ADF procedure is subjected to a nitrogen analysis giving the acid detergentinsoluble nitrogen (ADIN) as a percentage of total nitrogen.
Minerals
As stated earlier, most of the minerals found in plants are associated with organic compounds. Mineral analysis involves burning a sample of the feed, leaving an ash in which the minerals are present as simple inorganic (no carbon) salts. The ash is subsequently analyzed for each element.